Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kyono, Atsushi*; Yamamoto, Genichiro*; Yoneda, Yasuhiro; Okada, Satoru*
Isotope News, (783), p.23 - 27, 2022/10
Mineral traps are attracting attention as an underground storage method for carbon dioxide. Carbon dioxide laden groundwater reacts with basalt to form magnesite. The formed magnesium carbonate phase varies in many ways, but we tried to clarify the structure because all of them pass through amorphous magnesium carbonate. Pair distribution function using high-energy X-ray diffraction revealed that amorphous magnesium carbonate has a structure similar to that of hydromagnesite. It can be said that it is a safe sequestration method as a carbon dioxide storage technology.
 ambient on threshold voltage stability in 4H-SiC metal-oxide-semiconductor field-effect transistors
ambient on threshold voltage stability in 4H-SiC metal-oxide-semiconductor field-effect transistorsHosoi, Takuji*; Osako, Momoe*; Moges, K.*; Ito, Koji*; Kimoto, Tsunenobu*; Sometani, Mitsuru*; Okamoto, Mitsuo*; Yoshigoe, Akitaka; Shimura, Takayoshi*; Watanabe, Heiji*
Applied Physics Express, 15(6), p.061003_1 - 061003_5, 2022/06
Times Cited Count:2 Percentile:34.67(Physics, Applied)The combination of NO annealing and subsequent post-nitridation annealing (PNA) in CO ambient for SiO
ambient for SiO /SiC structures has been demonstrated to be effective in obtaining both high channel mobility and superior threshold voltage stability in SiC-based metal-oxide-semiconductor field-effect transistors (MOSFETs). N atoms on the SiO
/SiC structures has been demonstrated to be effective in obtaining both high channel mobility and superior threshold voltage stability in SiC-based metal-oxide-semiconductor field-effect transistors (MOSFETs). N atoms on the SiO side of the SiO
side of the SiO /SiC interface incorporated by NO annealing, which are plausible cause of charge trapping sites, could be selectively removed by CO
/SiC interface incorporated by NO annealing, which are plausible cause of charge trapping sites, could be selectively removed by CO -PNA at 1300
-PNA at 1300 C without oxidizing the SiC. CO
C without oxidizing the SiC. CO -PNA was also effective in compensating oxygen vacancies in SiO
-PNA was also effective in compensating oxygen vacancies in SiO , resulting high immunity against both positive and negative bias-temperature stresses.
, resulting high immunity against both positive and negative bias-temperature stresses.
 CO
CO on
on  C transfer to plants impacted by below-ground
C transfer to plants impacted by below-ground  CH
CH release
releaseOta, Masakazu; Tanaka, Taku*
Journal of Environmental Radioactivity, 201, p.5 - 18, 2019/05
Times Cited Count:4 Percentile:16.8(Environmental Sciences) CH
CH released from deep underground radioactive waste disposal facilities can be a belowground source of
released from deep underground radioactive waste disposal facilities can be a belowground source of  CO
CO owing to microbial oxidation of
owing to microbial oxidation of  CH
CH to
to  CO
CO in soils. Environmental
in soils. Environmental  C models assume that the transfer of
C models assume that the transfer of  CO
CO from soil to plant occurs via foliar uptake of
from soil to plant occurs via foliar uptake of  CO
CO . Nevertheless, the importance of
. Nevertheless, the importance of  CO
CO root uptake is not well understood. In the present study, belowground transport and oxidation of
root uptake is not well understood. In the present study, belowground transport and oxidation of  CH
CH were modeled and incorporated into an existing land surface
were modeled and incorporated into an existing land surface  CO
CO model (SOLVEG-II) to assess the importance of root uptake on
model (SOLVEG-II) to assess the importance of root uptake on  CO
CO transfer to plants. Performance of the model in calculating the belowground dynamics of
transfer to plants. Performance of the model in calculating the belowground dynamics of  CH
CH was validated by simulating a field experiment of
was validated by simulating a field experiment of  CH
CH injection into subsoil. The model was then applied to
injection into subsoil. The model was then applied to  C transfer in a hypothetical ecosystem impacted by continuous
C transfer in a hypothetical ecosystem impacted by continuous  CH
CH input from the water table (bottom of one-meter thick soil). In a shallowly rooted ecosystem with rooting depth of 11 cm, foliar uptake of
input from the water table (bottom of one-meter thick soil). In a shallowly rooted ecosystem with rooting depth of 11 cm, foliar uptake of  CO
CO was significant, accounting for 80% of the
was significant, accounting for 80% of the  C accumulation in the leaves. In a deeply rooted ecosystem (rooting depth of 97 cm), where the root penetrated to depths close to the water-table, more than half (63%) the
C accumulation in the leaves. In a deeply rooted ecosystem (rooting depth of 97 cm), where the root penetrated to depths close to the water-table, more than half (63%) the  C accumulated in the leaves was transferred by the root uptake. We found that
C accumulated in the leaves was transferred by the root uptake. We found that  CO
CO root uptake in this ecosystem depended on the distribution of methane oxidation in the soil; all
root uptake in this ecosystem depended on the distribution of methane oxidation in the soil; all  C accumulated in the leaves was transferred by the root uptake when methane oxidation occurred at considerable depths (e-folding depths of 20 cm, or 80 cm). These results indicate that
C accumulated in the leaves was transferred by the root uptake when methane oxidation occurred at considerable depths (e-folding depths of 20 cm, or 80 cm). These results indicate that  CO
CO root uptake contributes significantly to
root uptake contributes significantly to  CO
CO transfer to plants if
transfer to plants if  CH
CH oxidation occurs at great depths and roots penetrate deeply into the soil.
oxidation occurs at great depths and roots penetrate deeply into the soil.
Tamamura, Shuji*; Miyakawa, Kazuya; Aramaki, Noritaka*; Igarashi, Toshifumi*; Kaneko, Katsuhiko*
Groundwater, 56(1), p.118 - 130, 2018/01
Groundwater saturated with gas forms bubble under atmospheric pressure, bothering most authorized methods to determine in-situ dissolved gas concentrations. To circumvent this problem, a simple theory to quantify effect of 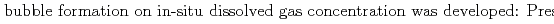 [bar]) of a gas component "
[bar]) of a gas component "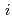 " (e.g., CH
" (e.g., CH , CO
, CO and H
and H ) in equilibrium with in-situ dissolved concentration (
) in equilibrium with in-situ dissolved concentration (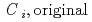 [mol L
[mol L  ]) was related to partial pressure of the
]) was related to partial pressure of the 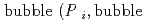 [bar]) emerged from groundwater in the form:
[bar]) emerged from groundwater in the form: 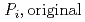 =
= 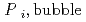 +
+ 
 (k
(k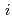 '
' 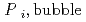 /(
/(
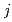 k
k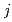 '
' 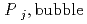 )), where
)), where 
 [bar] is groundwater pressure difference before and after the
[bar] is groundwater pressure difference before and after the 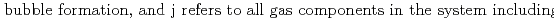 " The k
" The k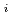 ' and k
' and k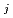 ' corresponds to the Henry's constant [bar L mol
' corresponds to the Henry's constant [bar L mol ] of
] of 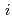 (k
(k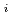 ) and
) and 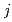 (k
(k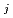 ), respectively, except for k
), respectively, except for k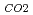 ', which is pH-dependent function. Dissolved CH
', which is pH-dependent function. Dissolved CH concentrations were successfully estimated by the model within the error of
concentrations were successfully estimated by the model within the error of  4.0% from the direct measurements by the sealed sampler method. Similar TIC concentration in the groundwater before and after the rm bubble formation was consistent with the model prediction. The wide application of the model is suggested without selecting sampling locations.
4.0% from the direct measurements by the sealed sampler method. Similar TIC concentration in the groundwater before and after the rm bubble formation was consistent with the model prediction. The wide application of the model is suggested without selecting sampling locations.
 emission reduction of active carbon recycling energy system for ironmaking by modeling with Aspen Plus
emission reduction of active carbon recycling energy system for ironmaking by modeling with Aspen PlusSuzuki, Katsuki*; Hayashi, Kentaro*; Kurihara, Kohei*; Nakagaki, Takao*; Kasahara, Seiji
ISIJ International, 55(2), p.340 - 347, 2015/02
Times Cited Count:19 Percentile:64.17(Metallurgy & Metallurgical Engineering)Use of the Active Carbon Recycling Energy System in ironmaking (iACRES) has been proposed for reducing CO emissions. To evaluate the performance of iACRES quantitatively, a process flow diagram of a blast furnace model with iACRES was developed using Aspen Plus, a chemical process simulator. CO
emissions. To evaluate the performance of iACRES quantitatively, a process flow diagram of a blast furnace model with iACRES was developed using Aspen Plus, a chemical process simulator. CO emission reduction and exergy analysis were performed by using mass and energy balance obtained from simulation results. The following CO
emission reduction and exergy analysis were performed by using mass and energy balance obtained from simulation results. The following CO reduction methods were evaluated as iACRES: solid oxide electrolysis cells (SOEC) with CO
reduction methods were evaluated as iACRES: solid oxide electrolysis cells (SOEC) with CO capture and separation (CCS), SOEC without CCS, and a reverse water-gas shift reactor powered by a high-temperature gas-cooled reactor. iACRES enabled CO
capture and separation (CCS), SOEC without CCS, and a reverse water-gas shift reactor powered by a high-temperature gas-cooled reactor. iACRES enabled CO emission reduction by 3-11% by recycling CO and H
emission reduction by 3-11% by recycling CO and H , whereas effective exergy ratio decreased by 1-7%.
, whereas effective exergy ratio decreased by 1-7%.
Hayashi, Kentaro*; Suzuki, Katsuki*; Kurihara, Kohei*; Nakagaki, Takao*; Kasahara, Seiji
Tanso Junkan Seitetsu Kenkyukai Saika Hokokusho; Tanso Junkan Seitetsu No Tenkai, p.27 - 41, 2015/02
Applying Active Carbon Recycling Energy System to ironmaking (iACRES) process is a promising technology to reduce coal usage and CO emissions. To evaluate performance of iACRES quantitatively, a process flow diagram of the blast furnace model with iACRES was developed using Aspen Plus. CO
emissions. To evaluate performance of iACRES quantitatively, a process flow diagram of the blast furnace model with iACRES was developed using Aspen Plus. CO emission reduction and exergy analysis was predicted by using mass and energy balance obtained from the simulation results. The followings were investigated as iACRES: solid oxide electrolysis cells (SOEC) with CO
emission reduction and exergy analysis was predicted by using mass and energy balance obtained from the simulation results. The followings were investigated as iACRES: solid oxide electrolysis cells (SOEC) with CO capture and separation (CCS), SOEC without CCS, and a reverse water-gas shift reactor as the a CO
capture and separation (CCS), SOEC without CCS, and a reverse water-gas shift reactor as the a CO reduction reactor powered by a high-temperature gas-cooled reactor. iACRES could provide CO
reduction reactor powered by a high-temperature gas-cooled reactor. iACRES could provide CO emission reductions of 3-11% by recycling CO and H
emission reductions of 3-11% by recycling CO and H , whereas the effective exergy ratio decreased by 1-7%.
, whereas the effective exergy ratio decreased by 1-7%.
Shu, Wataru; Ohira, Shigeru; Suzuki, Takumi; Nishi, Masataka
Fusion Science and Technology, 48(1), p.684 - 687, 2005/07
Times Cited Count:3 Percentile:24.22(Nuclear Science & Technology)As part of a series of studies on radiochemical reactions that may take place in the fuel processing systems of a future D-T fusion machine like the ITER, reactions of tritium molecule (T ) and carbon dioxide (CO
) and carbon dioxide (CO ) were examined by laser Raman spectroscopy and quadrupole mass spectrometry (QMS). Both T
) were examined by laser Raman spectroscopy and quadrupole mass spectrometry (QMS). Both T and CO
and CO decreased rapidly in the first 30 minutes after mixing, and then the reactions between them became much slower. As the predominant products of the reactions, carbon monoxide (CO) and tritiated water (T
decreased rapidly in the first 30 minutes after mixing, and then the reactions between them became much slower. As the predominant products of the reactions, carbon monoxide (CO) and tritiated water (T O) were found in gaseous phase and condensed phase, respectively. However, there existed also some solid products that were thermally decomposed to CO, CO
O) were found in gaseous phase and condensed phase, respectively. However, there existed also some solid products that were thermally decomposed to CO, CO , T
, T , T
, T O, etc. during baking at 150
O, etc. during baking at 150 C and 250
C and 250 C.
C.
Meguro, Yoshihiro; Tomioka, Osamu; Imai, Tomoki*; Fujimoto, Shigeyuki*; Nakashima, Mikio; Yoshida, Zenko; Honda, Tadashi*; Koya, Fumio*; Kitamura, Nobu*; Wada, Ryutaro*; et al.
Proceedings of International Waste Management Symposium 2004 (WM '04) (CD-ROM), 8 Pages, 2004/03
Supercritical CO fluid leaching (SFL) method using supercritical CO
fluid leaching (SFL) method using supercritical CO fluid containing a complex of HNO
fluid containing a complex of HNO - tri-n-butyl phosphate (TBP) was applied to removal of uranium from radioactive solid wastes. Sea sands, incineration ashes and porous alumina bricks were employed as matrixes of simulated solid wastes. Real radioactive incineration ash wastes and firebrick wastes were also subjected to the SFL treatment. It was found that uranium could be efficiently removed from both of the simulated wastes and real wastes by the SFL method. The removal efficiency of uranium from the real waste was lower than that from the corresponding artificial waste. About 1 g and 35 mg of uranium were recovered from 10 g of the real ash waste and 37 g of the real firebrick waste, respectively.
- tri-n-butyl phosphate (TBP) was applied to removal of uranium from radioactive solid wastes. Sea sands, incineration ashes and porous alumina bricks were employed as matrixes of simulated solid wastes. Real radioactive incineration ash wastes and firebrick wastes were also subjected to the SFL treatment. It was found that uranium could be efficiently removed from both of the simulated wastes and real wastes by the SFL method. The removal efficiency of uranium from the real waste was lower than that from the corresponding artificial waste. About 1 g and 35 mg of uranium were recovered from 10 g of the real ash waste and 37 g of the real firebrick waste, respectively.
Watanabe, Takeshi*; Tsushima, Satoru*; Yamamoto, Ichiro*; Tomioka, Osamu; Meguro, Yoshihiro; Nakashima, Mikio; Wada, Ryutaro*; Nagase, Yoshiyuki*; Fukuzato, Ryuichi*
Proceedings of 2nd International Symposium on Supercritical Fluid Technology for Energy and Environment Applications (Super Green 2003), p.363 - 366, 2004/00
Recovery of salts by supercritical fluid leaching (SFL) method using carbon dioxide was experimentally studied. It was confirmed that LiCl was recovered with a mixed fluid of carbon dioxide and methanol, and KCl and SrCl were recovered with a mixed fluid of carbon dioxide, methanol and crown ether. The influence of crown ether for KCl and SrCl
were recovered with a mixed fluid of carbon dioxide, methanol and crown ether. The influence of crown ether for KCl and SrCl extraction were found to increase in the order of 15-crown-5 (15C5)
extraction were found to increase in the order of 15-crown-5 (15C5)  18-crown-6 (18C6)
18-crown-6 (18C6)  dicychlohexyl-18-crown-6 (DC18C6). It is expected that other salts can be recovered selectively with a mixed fluid of carbon dioxide, methanol and suitable crown ether.
dicychlohexyl-18-crown-6 (DC18C6). It is expected that other salts can be recovered selectively with a mixed fluid of carbon dioxide, methanol and suitable crown ether.
Nagase, Yoshiyuki*; Masuda, Kaoru*; Wada, Ryutaro*; Yamamoto, Ichiro*; Tomioka, Osamu; Meguro, Yoshihiro; Fukuzato, Ryuichi*
Proceedings of 2nd International Symposium on Supercritical Fluid Technology for Energy and Environment Applications (Super Green 2003), p.254 - 257, 2004/00
no abstracts in English
Hakoda, Teruyuki; Hashimoto, Shoji; Kojima, Takuji
Bulletin of the Chemical Society of Japan, 75(10), p.2177 - 2183, 2002/10
Times Cited Count:8 Percentile:37.18(Chemistry, Multidisciplinary)no abstracts in English
 -diketone and tributylphosphate
-diketone and tributylphosphateMeguro, Yoshihiro; Iso, Shuichi; Ogiyanagi, Jin; Yoshida, Zenko
Analytical Sciences (CD-ROM), 17(Suppl.), p.721 - 724, 2002/03
no abstracts in English
 /supercritical CO
/supercritical CO +TBP system
+TBP systemMeguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko
Proceedings of International Solvent Extraction Conference 2002 (CD-ROM), p.1131 - 1136, 2002/00
no abstracts in English
 medium containing HNO
medium containing HNO -TBP complex
-TBP complexTomioka, Osamu*; Meguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko; Enokida, Yoichi*; Yamamoto, Ichiro*
Journal of Nuclear Science and Technology, 38(6), p.461 - 462, 2001/06
Times Cited Count:27 Percentile:85.72(Nuclear Science & Technology)no abstracts in English
Clifford, A. A.*; Zhu, S.*; Smart, N. G.*; Lin, Y.*; Wai, C. M.*; Yoshida, Zenko; Meguro, Yoshihiro; Iso, Shuichi
Journal of Nuclear Science and Technology, 38(6), p.433 - 438, 2001/06
Times Cited Count:14 Percentile:68.97(Nuclear Science & Technology)no abstracts in English
Ogawa, Masuro
Enerugi, 34(5), p.81 - 87, 2001/05
no abstracts in English
Meguro, Yoshihiro
Bunseki, 2001(4), p.187 - 188, 2001/04
no abstracts in English
Ohashi, Hirofumi; Sakaki, Akihiro; Inagaki, Yoshiyuki
JAERI-Research 2000-058, 64 Pages, 2001/01
no abstracts in English
 , CO
, CO and N
and N O in Tokai-mura
O in Tokai-muraPorntepkasemsan, B.*; Atarashi-Andoh, Mariko; Amano, Hikaru
JAERI-Data/Code 2000-032, 43 Pages, 2000/11
no abstracts in English
Mine, Tatsuya*; Mihara, Morihiro;
JNC TN8430 2000-010, 27 Pages, 2000/07
In the geological disposal system of the radioactive wastes, gas generation by microorganism could be significant for the assessment of this system, because organic material included in groundwater, buffer material and wastes might serve as carbon sources for microorganisms. In this study, gas generation tests using microorganisms were carried out under anaerobic condition. The amount of methane and carbon dioxide that were generated by activity of Methane Producing Bacteria (MPB) were measured with humic acid, acetic acid and cellulose as carbon sources. The results showed that methane was not generated from humic acid by activity of MPB. However, in the case of using acetic acid and cellulose, methane was generated, but at high pH condition (pH=10), the amount of generated methane was lower than at low pH (pH=7). It was not clear whether the pH would affect the amount of generated carbon dioxide.